
The neutralization reaction is also known as the acid base neutralization reaction, or just the acid base reaction.
We define neutralization as the chemical reaction between an acid and a base. The acid and base are reacted away, and neither remains. In their place, pure, neutral water forms. Hence the name neutralization. Plus a salt product forms as well.
Below are 2 neutralization reaction examples. Learn to solve the acid base neutralization reaction the easy way… using the inner-outer pair rule.
The neutralization reaction worksheet, with answer key, is at the bottom of this page.
Do the acid base neutralization reaction worksheet below if you want to really learn how acid base neutralization reactions work. It’s a single, printer-friendly page. Print it and write out the answers. Or just write out the reactions on a blank piece of paper. We promise you will learn more by writing out the answers, not just looking at the worksheet!
You can check your acid base neutralization reaction worksheet answers using the answer key.
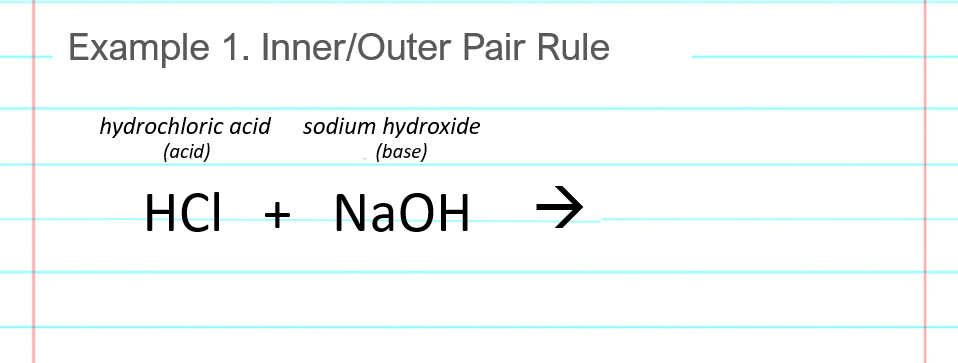
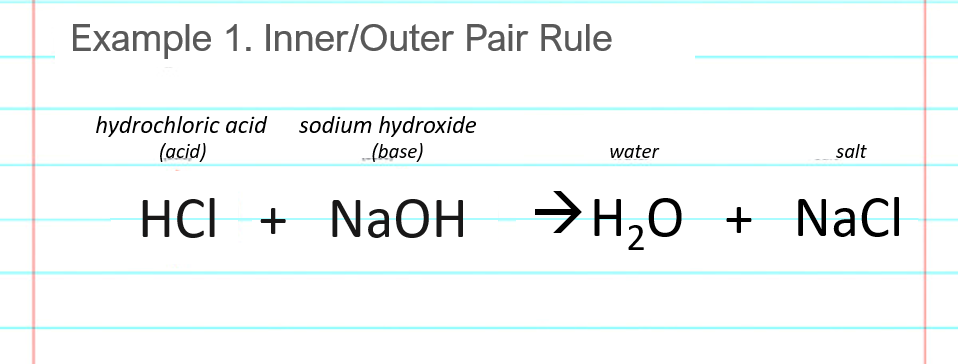
Neutralization reaction example #1 shows the acid base reaction for hydrochloric acid and sodium hydroxide. The acid and base are HCl and NaOH, respectively.
The below steps show how to use the inner-outer pair rule to find the products.
Write down the reactants, one acid and one base, in any order. (HCl + NaOH or also NaOH + HCl is fine.) The plus arrow between the acid and base means that they are mixed together, and the arrow indicates that a chemical reaction occurs. Our goal is to find the products that are formed in the chemical reaction. They go in the blank space to the right of the arrow. You don’t have to write the names as shown above the chemical formulas in the example.
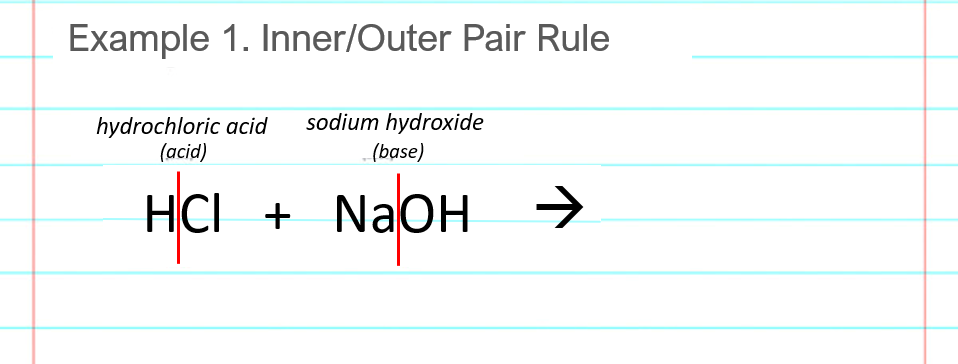
Split or break the acid and base after the first atom. This is shown as the vertical red lines in the below image. The reactants to the left of the arrow must break apart, so that the parts can rearrange to form the products that go on the right of the arrow.
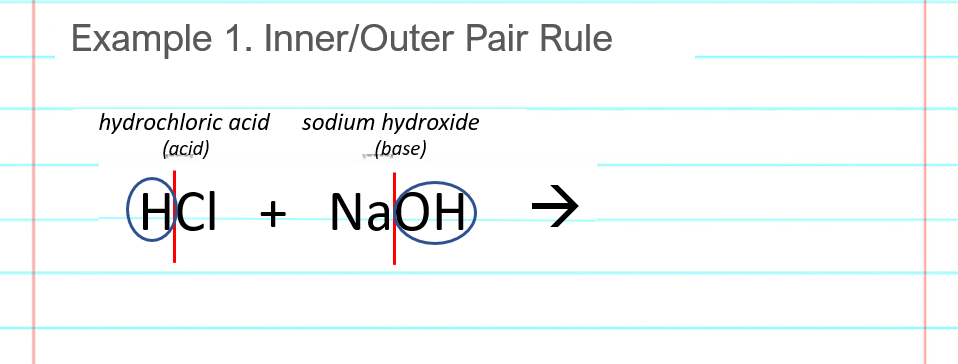
Find the “outer pair” of atoms, the ones on the outsides of the red lines from the previous step. In this example, the outer pair is an H and OH which combine to make HOH, or H2O, water.
Find the “inner pair” of atoms, the ones on the insides of the red lines from the previous step. In this example, the inner pair is a Cl and an Na, which combine to make NaCl, salt. Note the need to switch the atoms around, as Na is a first part and Cl is a second part. It’s NaCl, not ClNa. Another way to think, if you have a periodic table handy, is that the element to the left in the periodic table goes to the left in the formula.
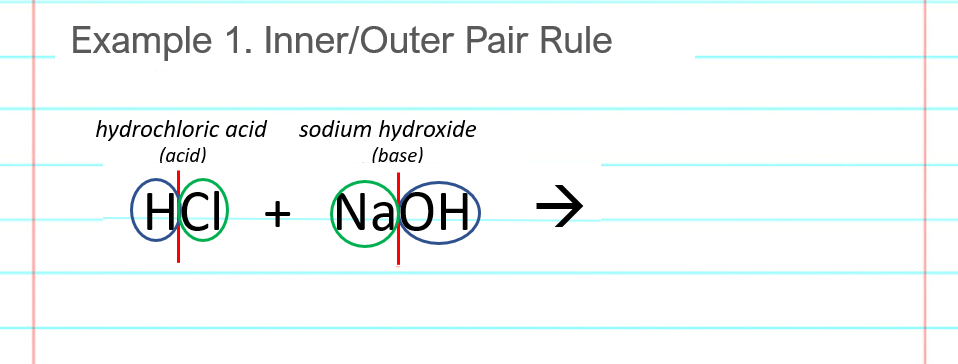
Write the products cleanly to the right of the arrow where they belong. This says that the hydrochloric acid and sodium hydroxide (HCl and NaOH) combine to produce water (H2O) and salt (NaCl). You can write the products in any order (NaCl + H2O is fine too).
The lines, circles, and pairs shown above are just your work to guide you through the problem. With some practice, you can visualize the inner outer pair rule mentally and just write down the products.
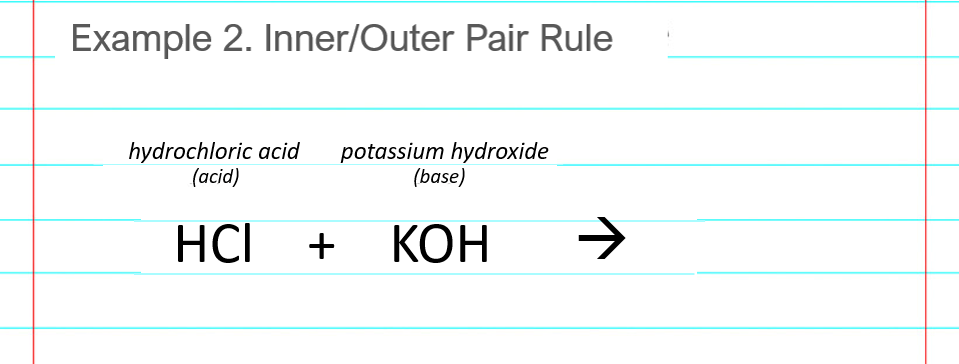
Neutralization reaction example #2 shows the acid base reaction for hydrochloric acid and potassium hydroxide. The acid and base are HCl and KOH, respectively.
The below steps show how to use the inner-outer pair rule to find the products.
Write down the reactants in any order. (KOH + HCl is also fine.)
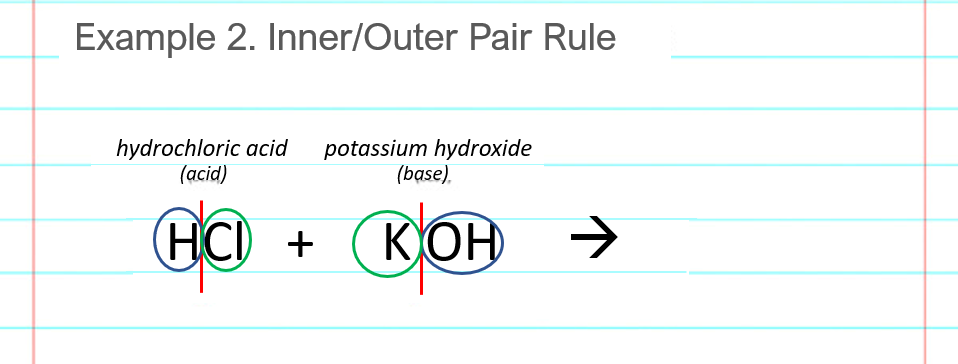
Split or break the acid and base after the first atom (see red lines below). Find the “outer pair” of atoms, in this example an H and an OH which combine to make HOH, or H2O, water. Find the “inner pair” of atoms, in this example a Cl and a K which combine to make KCl (not ClK, switch the letters around). You could also do the inner pair before and the outer pair, as the order doesn’t matter.
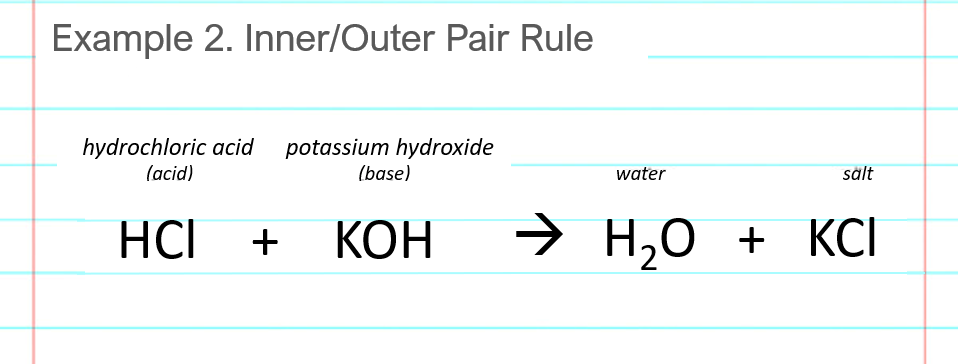
Write the products cleanly to the right of the arrow where they belong. You can write the products in any order (KCl + H2O is fine too).
Here are a few nit picky details, in case you are interested to learn more.
1. The inner-outer pair rule is a trick that only works for certain types of problems. The acid must start with a single letter H and the base must end in OH. Technically speaking, the trick works for reactions between an Arrhenius, monoprotic acid and a strong base. For other substances, like diprotic acids, triprotic acids, organic acids, and weak bases, you’ll need to know more about chemistry. Still, the inner-outer pair rule as shown above is your best option to start learning about acid-base chemistry. It all builds from here. Did you find the neutralization reaction worksheet answers?
2. Neutralization is a chemical reaction between an acid and a base. Neutralization does not necessarily make a neutral (pH = 7) solution, even though it forms some neutral water. In all likelihood, a neutralization reaction will produce a non-neutral solution. We define neutralization and neutral quite differently in chemistry, and they are definitely NOT the same thing.