Resonance Structures
What is a Resonance Structure?
Resonance structures happen when there are multiple, correct Lewis structures for a molecule.
There must be a double bond, and there must be more than one way to draw the double bond. Basically, like a fork in the road, there is a choice to me made when drawing the molecules.
It’s easier to see resonance than explain it in words. There are clear examples below for SO2 and ozone.
O3 Resonance Structures
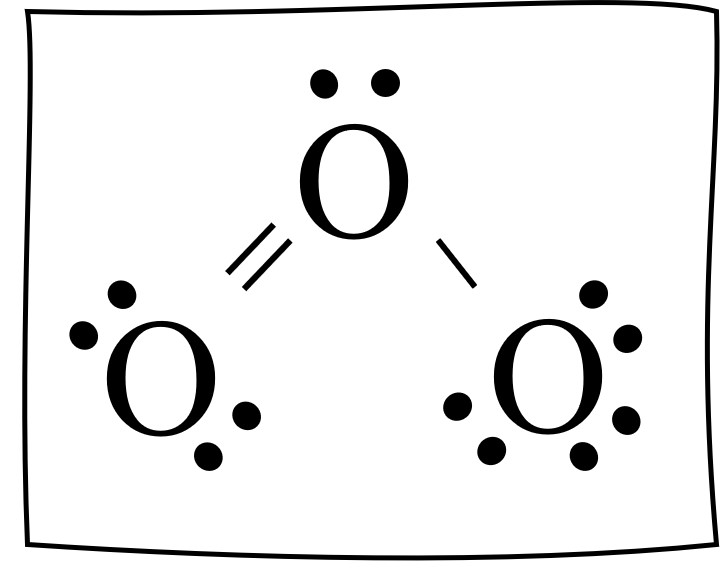
The ozone Lewis structure is shown below. This is a correct Lewis structure. There is a central oxygen atom (O) with: a singled bonded oxygen atom, a double bonded oxygen atom, and a pair of dots shown on top.
Notice how the double bond goes left and the single bond goes right. Would it work the other way? Of course.
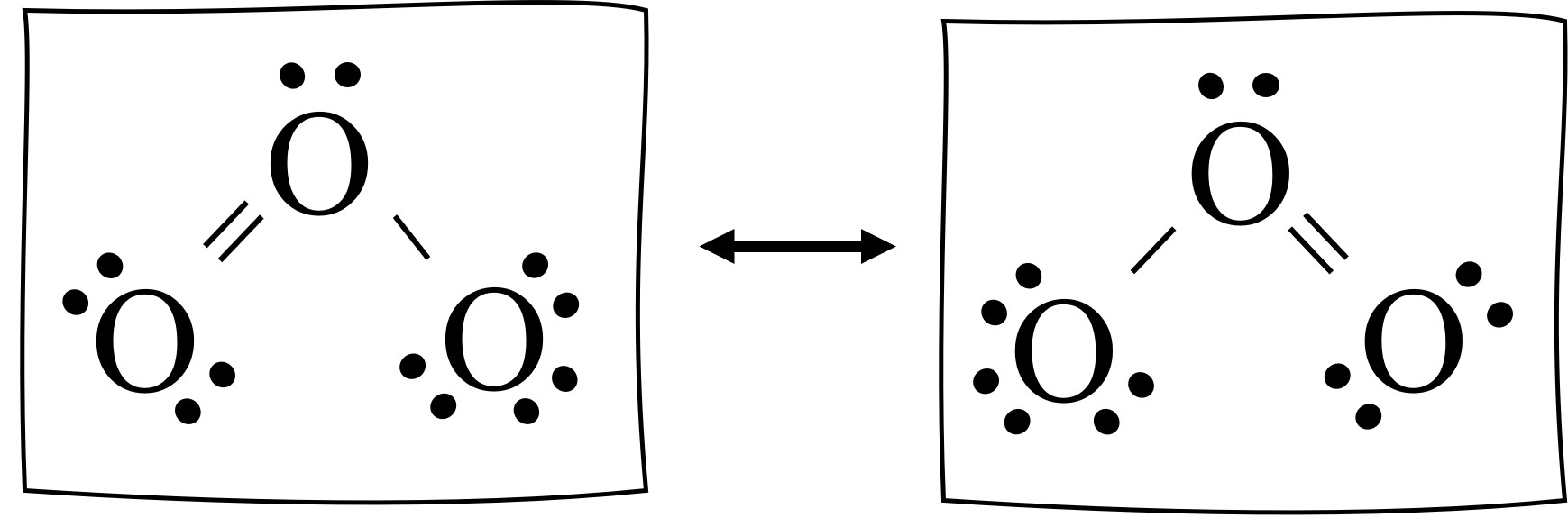
The below image shows both ways to draw the ozone Lewis structure. With a double pointed arrow between the diagrams. The arrow indicates that the molecules is switching back and forth (resonating) between the two options. It spends about 50% of the time with the double bond left, and about 50% of the time with the double bond right.
The entire figure below is the resonance structure for ozone. The resonance structure includes both Lewis structures as well as the double arrow between them. This is how we show resonance in chemistry.
Resonance Hybrid vs. Resonance Structures
The above O3 resonance structures are real, as opposed the the resonance hybrid, which is not. The resonance hybrid means the average of the above two resonance structures. This average Lewis structure is, by definition, a mathematical artifact that is not real. We could say, on average, there are 1.5 bonds going left and 1.5 bonds going right. We could say the average bond order is 1.5.
But, any real, actual resonance structure has a double bond one way and a single bond the other. It’s mostly semantics, yet we say that resonance structures are real while resonance hybrids are purely mathematical in nature.
SO2 Resonance Structures
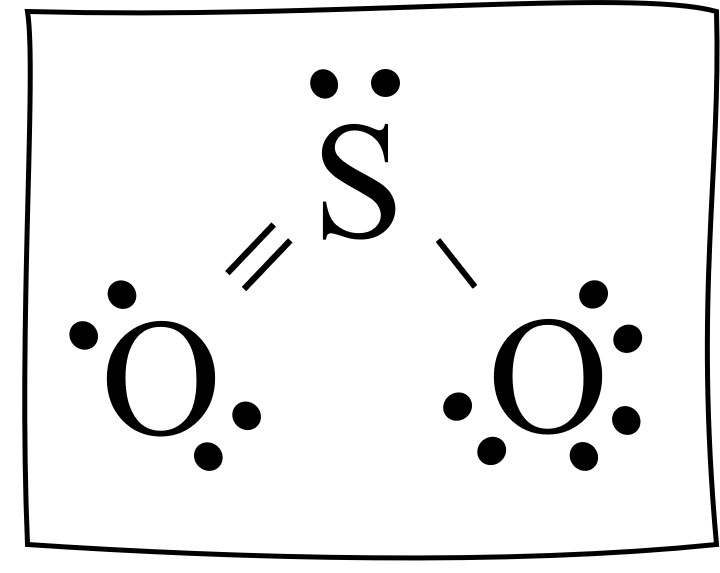
The sulfur dioxide SO2 Lewis structure is shown below. This is a correct Lewis structure. There is a central sulfur atom (S) with: a singled bonded oxygen atom, a double bonded oxygen atom, and a pair of dots shown on top.
Would it work the other way, with the double bond going right? Again, of course it would.
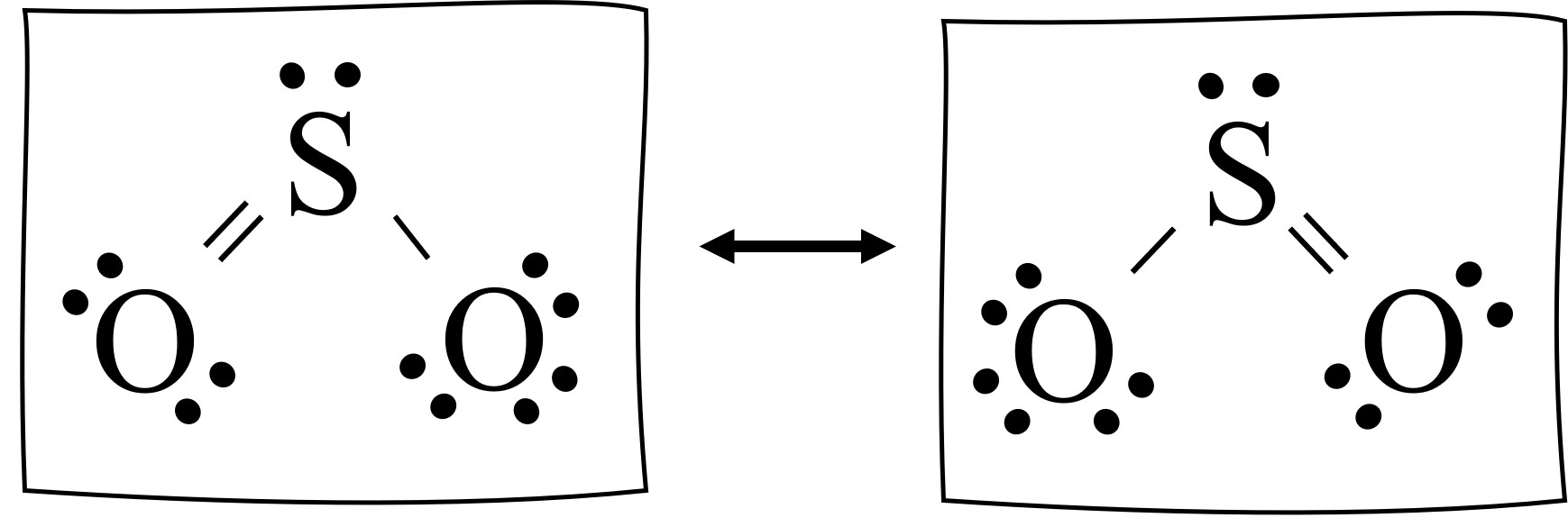
The below image shows the SO2 resonance structures. There are two resonance structures, and SO2 spends half it’s time in each. It flips back and forth rapidly (resonates) between the two forms.
The pair of resonance structures represent two resonance forms of SO2 that physically exist in time and space. Resonance structures are taken to be real, as they are not just artifacts that result from drawing the molecules.
Resonance Definition
A formal resonance definition requires some more vocabulary. In the above examples, the double bond is shifting from right to left. This represents a pair of electrons that are moving around inside the molecule. Such electrons are said to be delocalized.
A formal way to define resonance is as a way to draw molecules that have delocalized electrons.
So what is a resonance structure, really? It just means there are multiple correct drawings, known as resonance forms, which are taken to be real. The delocalization of electrons is also real, however, and these delocalized electrons are often the key to explaining how the chemical will react. The delocalized electrons are highly mobile and thus often highly reactive.
Resonance and delocalization are frequently important topics for understanding organic chemistry, yet these topics are typically taught in inorganic chemistry courses.

