Solubility Table
Download the Solubility Table
Download and print the black and white pdf. It’s 1 printer-friendly page.
Prefer YouTube style video lessons? Get unlimited access for a low monthly membership fee.
Solubility Definition
Solubility means “dissolvability,” except that “dissolvability” isn’t a proper science word. The proper solubility definition is the ability to dissolve. Soluble things dissolve, and insoluble things don’t dissolve.
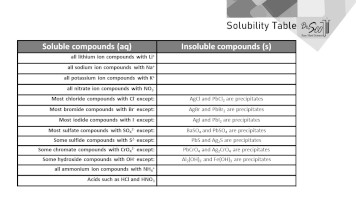
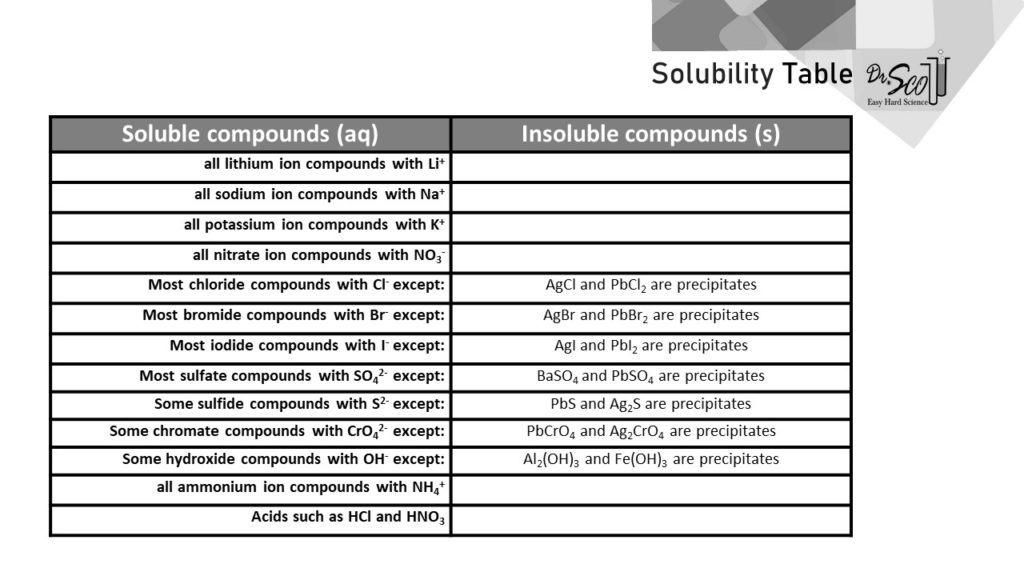
When you put something solid into water, there are exactly two possible outcomes. It either dissolves or it doesn’t. The purpose of the Solubility Table is to tell whether a substance will dissolve or not. If a substance dissolves in water, we say it forms an aqueous solution denoted (aq) in chemistry. If a solid substance substance does not dissolve, it remains solid denoted (s) in chemistry.
The Solubility Table has two columns. The left column indicates substances that dissolve in water, denoted as soluble substances or (aq). The right column indicates substances that won’t dissolve in water, denoted as insoluble substances or (s). Note that the Solubility Table doesn’t use the word “dissolve,” which would make things a bit more obvious. A common mistake is thinking that (s) is for soluble when instead (s) stands for solid, which would be an insoluble substance that doesn’t dissolve.
If an insoluble substance (that won’t dissolve) forms in a reacting solution, we say the substance is a precipitate. So, if you are working out a precipitation reaction in chemistry, the right column is also a list of the possible precipitates.
What is the basis of the Solubility Table? First, it’s based on experimental data of what actually happens. Theoretically, if a substance is soluble, it means it has a strong force of attraction to water. That’s why it dissolves, because it’s atoms like to stick to water. If a substance is insoluble, it is because it has a stronger force of attraction to itself as compared to water.
Solubility Table Examples
Note that other sources may have additional information. This is a simplified table to demonstrate how the solubility rules work. The below 3 examples show the 3 basic cases: soluble, insoluble, and undetermined.
Example 1: Is NaCl Soluble in Water?
Yes. The second row, first column in the Solubility Table says that all compounds with sodium ion Na+ are soluble. This means that NaCl is soluble in water. It dissolves.
Note that to determine if NaCl is soluble in water, you do need to recognize that it in an ionic compound made of sodium ions Na+ and chloride ions Cl–. Review the names and formulas for ionic compounds if you are unclear on this.
Furthermore, the fifth line of the Solubility Table says that most compounds with chloride Cl– are soluble. There are some exceptions invoving silver and lead in the right column, but this doesn’t fit NaCl. For a second reason, NaCl is clearly soluble or (aq).
Example 2: Is BaSO4 Soluble or Insoluble?
Insoluble. The eighth row about sulfate compounds says, in the right column, that BaSO4 is specifically an insoluble compound. This compound, barium sulfate, remains solid (s) in water and is a common precipitate.
Note that whether is BaSO4 soluble or insoluble requires some knowledge of polyatomic ions. Review sulfate and other polyatomics if you are unclear of this.
Also note the above Solubility Table is specifically hiding some important information. It so happens that barium sulfate does precipitate at low temperatures, but it will not precipitate at higher temperatures much above room temperature. So, is BaSO4 soluble or insoluble? Well, it really depends on the temperature. The point here is that some Solubility Tables show the temperature dependence but most do not. So you really have to be careful using a Solubility Table!
Example 3: Is CaCO3 Soluble in Water?
No. It’s an insoluble precipitate. But the above Solubility Table says nothing about carbonate compounds one way or the other. It’s impossible that any table could have all possible compounds, and here is an example where the information is missing altogether from the table. It’s unfortunate yet all too common that the Solubility Table just doesn’t have the info you are looking for.
So, is CaCO3 soluble in water? Nope. It precipitates to form stalactites, stalagmites, rock columns, and even spectacular petrified waterfalls. Also, see solubility rule #7 below for a better explanation.
Solubility Rules
The Solubility Table is sometimes also shown as the Solubility Rules. The solubility rules are meant to have the same information as the table, yet as we know all tables are a bit different. Hence you may see various solubility rules as well.
If you look close, you will see the above Solubility Table says the same thing as the below solubility rules. Except that rule #5 discusses substances that are soluble vs insoluble vs slightly soluble. The term “slightly soluble” means that it’s neither clearly soluble nor insoluble. In the real world it’s just really not so simple as having just two, clear cut cases.
Solubility Rules
- Salts with an element from Column 1 such as Li+, Na+, and K+ are soluble. Likewise, ammonium ion (NH4+) compounds are soluble.
- Salts with nitrate NO3– are generally soluble.
- Salts with a halide (Cl–, Br–, and I–) are usually soluble, except compounds with silver or lead (II) ions are insoluble.
- Most sulfates (SO42-)are soluble, except BaSO4 and PbSO4 are insoluble.
- Hydroxides (OH–) are soluble with Column 1 metals; slightly soluble* with Column 2 metals; and insoluble with transition metals or aluminum ion, such as Al2(OH)3 and Fe(OH)3.
- Sulfides (S2-) of transition metals are highly insoluble, such as Ag2S and PbS.
- Carbonates such as CaCO3 are insoluble.
- Chromates such as PbCrO4 and Ag2CrO4 are insoluble.