The O2 Lewis structure has a double bond between two oxygen atoms. According to the octet rule, oxygen atoms need to bond twice.
The O2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
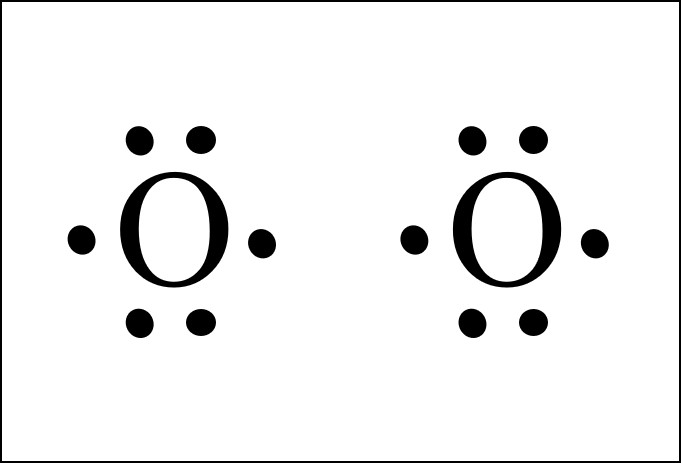
It’s easiest to think in terms of dots to make the O2 Lewis structure.
Oxygen needs to bond twice, shown as the lone dots on the left and right sides of the oxygen atoms in the below diagram. There are also two pairs of dots, representing four more electrons, that won’t bond.
Think of connecting the lone dots to form bonds between each O atom. Each O atom needs to bond twice. So the pair of O atoms form two bonds with each other.
The two bonds appear as the two parallel lines between the O atoms. This is called a double bond. Each bond is a pair of electrons, one from each connected O atom. So the double bond, the two parallel lines, represents a total of 4 electrons.
Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in the O2 double bond. So each O is surrounded by 8 total valence electrons, giving it an octet and making it stable.
The two letter O’s in the O2 Lewis structure represent the nuclei (centers) of the oxygen atoms. The nuclei contain the protons and neutrons, which are the solid parts of the molecule. Interestingly, the dots and lines represent electrons, which are not solid. The diagram is drastically out of scale, as the relative size of the nucleus compared to the surrounding electrons is usually comparable to a pea in a stadium.
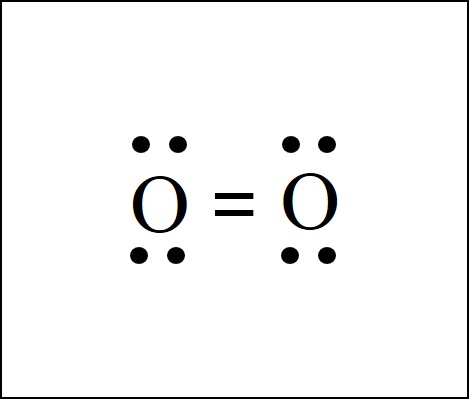
The O2 Lewis structure shows two oxygen atoms bonded in the same way to each other. It’s perfectly symmetric.
Generally, small symmetric molecules are nonpolar. The O2 Lewis structure indicates that the O2 molecule is perfectly symmetric. Therefore, O2 is a nonpolar substance.
Small nonpolar substances tend to be gasses. They tend to have low boiling points. For example, O2 must be chilled to about -180 ℃ or -300 ℉ to liquify it. The Earth does not get this cold, and the atmosphere stays filled with O2 gas.