Octet Rule
What is the Octet Rule?
The octet rule is a concept in chemistry. It allows us to determine the atomic structure of most chemicals. The octet rule is also used in determining the names and formulas for many chemicals.
As you might suspect, the octet rule is based on the number 8. It is a direct consequence that the periodic table has 8 main columns (the ones near the top).
Octet Rule & The Periodic Table
The periodic table is shown below with the main 8 columns of the periodic table. These “main” columns are the ones near the top of the table.
The number 1-8 indicates the number of valence electrons for some atom. Atoms are unstable unless they have 8 valence electrons. Thus, only the noble gasses are “born” to be stable. All other atoms must gain, lose, or share electrons to be more like a noble gas. Stable atoms tend to have 8 valence electrons, like the noble gasses.
The elements in the lower columns in the middle do not have numbers, and they typically don’t obey the octet rule.
Octet Rule & Covalent Bonds
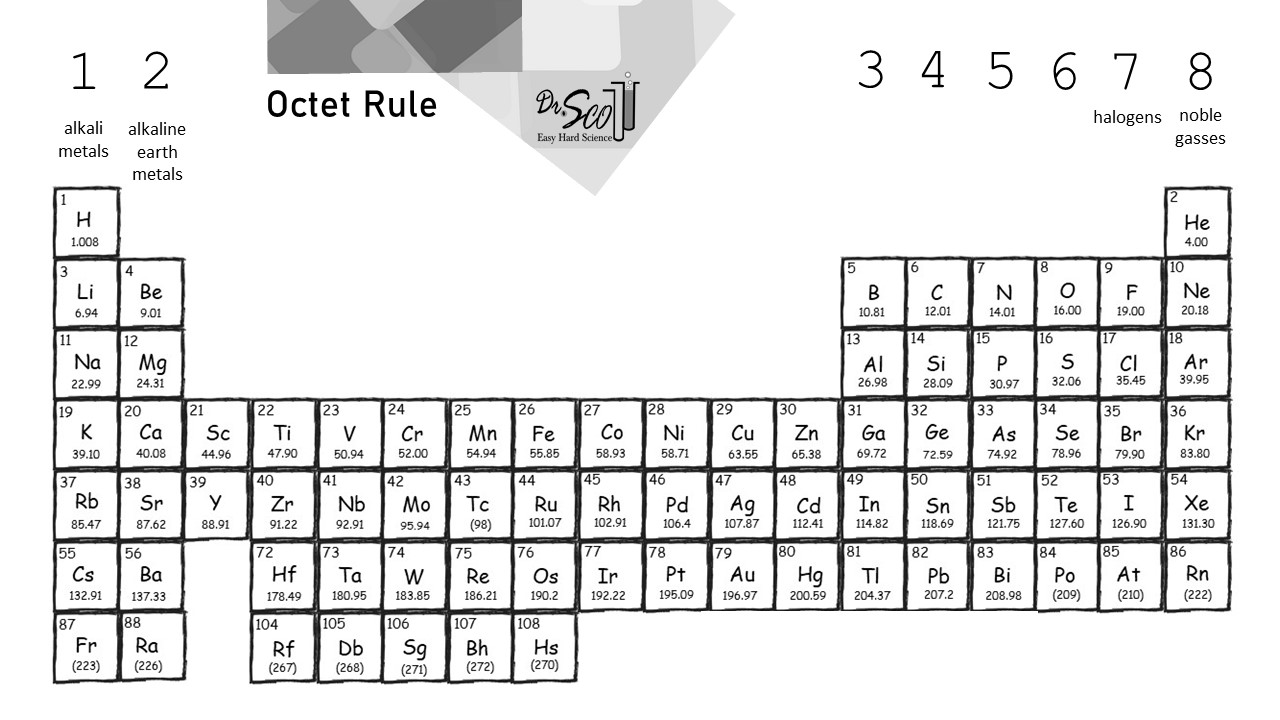
Molecules are formed when atoms make covalent bonds. Each bond allows an atoms to get 1 additional electron, moving it closer to an octet. Only the nonmetal elements in columns 4, 5, 6, and 7 form covalent bonds. Metals form other types of bonds, and the noble gasses don’t form bonds.
How Many Valence Electrons for Carbon?
Carbon (symbol C) is found in column 4 on the periodic table. It has 4 valence electrons. It needs to make 4 bonds to get an octet. The simple logic is that 4 + 4 = 8.
In the diagram above, we show carbon making 4 bonds. The “C” represents the carbon nucleus (center), and the 4 lines (or sticks) going up, down, left, and right represent the bonds.
The same idea would apply to silicon (Si), another element in column 4.
How Many Valence Electrons Does Nitrogen Have?
Nitrogen (symbol N) is found in column 5 on the periodic table. It has 5 valence electrons. It needs to make 3 bonds to get an octet. The simple logic is that 5 + 3 = 8.
In the diagram above, we show nitrogen making 3 bonds. These are the 3 sticks/lines are N. Each stick or line represents an electron that N will use to bond. The 3 bonds represent 3 electrons, but there are 5 valence electrons total. Hence the pair of dots over N representing a pair of electrons that will not bond. The dots could be drawn left, down or right, yet they are usually shown above the N.
The same idea would apply to phosphorus (P), another element in column 5.
How Many Electrons Does Oxygen Have?
Oxygen (symbol O) is found in column 6 on the periodic table. It has 6 valence electrons. It needs to make 2 bonds to get an octet. The simple logic is that 6 + 2 = 8.
In the diagram above, we show oxygen making 2 bonds. The 2 sticks/lines account for 2 of the 6 valence electrons. The other 4 valence electrons are found as the two pairs of dots that won’t bond.
The same idea would apply to sulfur (S), another element in column 6. There are notable exceptions to the octet rule, however, such as the ability of sulfur to form 6 bonds (not shown here).
How Many Valence Electrons Does Fluorine Have?
Fluorine (symbol F) is found in column 7 on the periodic table. It has 7 valence electrons. It needs to make 1 bond to get an octet. The simple logic is that 7 + 1 = 8.
In the diagram above, we show fluorine making 1 bond. The bond could be drawn up, down, left, or right. There are 6 more fluorine valence electrons appearing as 3 pairs of dots. The fluorine valence electrons appearing as dots won’t bond.
The same goes for the other halogens. How many electrons does chlorine have? There are 7 valence electrons for chlorine, and it would have 1 bond and three pairs of dots. Same for bromine (Br) and iodine (I).
Octet Rule for the Noble Gasses
The noble gasses are noble. They don’t bond. They are already born with 8 electrons, generally, so they already obey the octet rule without bonding. The simple logic is that 8 + 0 = 8 and they won’t bond.
The figure above shows neon (Ne) surrounded by 4 pairs of dots, without any bonds. The same would apply for Ar, Kr, and Xe.
There are more exceptions to the octet rule for the noble gasses. Note that helium (He) is noble. It is element number 2 with only 2 electrons, so it cannot possibly have an octet of 8 electrons. Still, helium is noble, and elements near helium on the periodic table are stabilized with 2 electrons (not an octet of 8). What’s really important is having an electron configuration like a noble gas… the octet rule and the number 8 are secondary to this.
Speaking of exceptions to the octet rule, apparently somebody made XeF2 in a fancy lab. Xenon is a noble gas and isn’t expected to bond. Yet it did, apparently twice.
Exceptions to Octet Rule
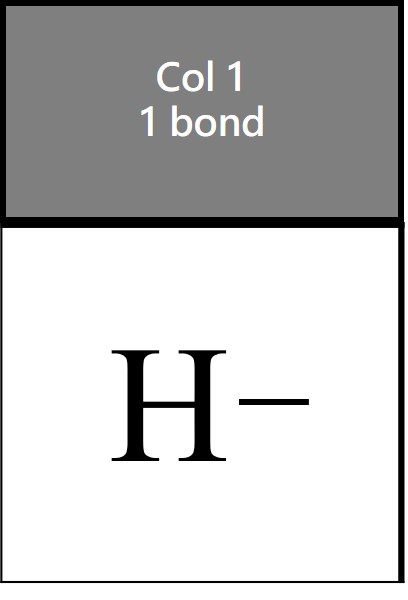
How Many Valence Electrons Does Hydrogen Have?
Hydrogen is very different than the other elements. Interestingly, it’s the most plentiful element in the universe.
Hydrogen is another of the exceptions to the octet rule. It is born with 1 electron. It takes an electron to form a bond. So hydrogen just forms a bond. It is drawn as an H with one stick or line. That’s it. No dots showing any hydrogen valence electrons.
The hydrogen valence electrons act like helium, element number 2. There is 1 hydrogen valence electron and H forms 1 bond. The simple logic is that 1 + 1 = 2 for hydrogen. Helium and hydrogen are exceptions to the octet rule, and some say the “duet rule” applies. H and He are stabilized with 2 electrons, not an octet. (Duet means 2).
Chlorine Dioxide and the Octet Rule: Good or Bad?
Chlorine dioxide is a good example of a molecule that doesn’t obey the octet rule. The formula for chlorine dioxide is ClO2, and it’s generally taken to mean the chlorine is bonded twice, instead of the usual once. That doesn’t make sense in terms of the octet rule, as we’d get something along the lines of 7 + 2 = 9 when counting the chlorine valence electrons. Arg. But it’s a real molecule, chlorine dioxide. Not all molecules obey the octet rule.
Chlorine dioxide is a bit special in not following the octet rule. The odd electron count means that there must be an unpaired electron. This unpaired electron accounts for the rather high reactivity of ClO2. As such, chlorine dioxide uses include industrial oxidants and disinfects for drinking water and food. The U.S. Food and Drug Administration describes generation and application of this fairly common chemical in 21CFR173.300. So, it’s failure to obey the octet rule doesn’t make chlorine dioxide bad, rare or useless… it’s a fairly common, important and useful chemical.
Sulfur Hexafluoride Disobeys the Octet Rule
Sulfur hexafluoride (SF6) is another molecule that doesn’t obey the octet rule. It has sulfur S bonded to six fluorine atoms F. With two electrons per bond, that makes 12 electrons for sulfur. That’s a bit more than an octet of 8. So, as a rule, sometimes sulfur can form 6 bonds instead of the normal 2.
Sulfur hexafluoride, or SF6, is a real chemical. It’s used as an electrical insulator inside high voltage equipment. Although non-toxic to breathe, SF6 can react to form harmful substances when exposed to certain types of electric discharges. It also acts as a greenhouse gas when it eventually leaks from electrical equipment into the atmosphere. Although it lacks an octet, which usually makes molecules stable, SF6 is extremely stable. Sulfur hexafluoride is estimated to float around the atmosphere for centuries. Now that’s a stable molecule!
The above describes how the octet rule applies to covalent bonds, as found in molecules. There is a whole second case for how the octet rule apples for ionic bonds.
Click here to learn about ionic compounds and the other case for using the octet rule.
