Diatomic Elements
Diatomics on the Periodic Table
The 7 diatomic elements are hydrogen (H), nitrogen (N), oxygen (O), fluorine (F), chlorine (Cl), bromine (Br), and iodine (I).
We call them diatomic elements because the atoms appear in pairs. The chemical formulas for these elements are H2, N2, O2, F2, Cl2, Br2, and I2.
The diatomic elements are easy to find on the periodic table. They include the halogens (F, Cl, Br, I) plus O and N. These elements are touching on the periodic table. Hydrogen is apart from the other diatomics on the periodic table.
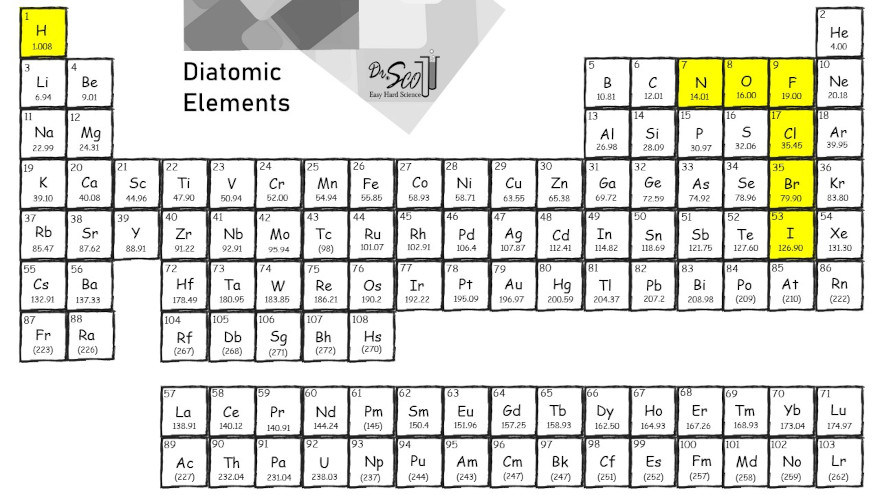
Diatomic Element Structure
The diatomic elements are elements that appear as molecules. There is a pair of atoms with a chemical bond. Dot structures for F2, Cl2, Br2, I2, and H2 are shown below. The horizontal line represents a bond between the pair of atoms, indicated by the letters. The atoms are more stable in pairs because they obey the octet rule.
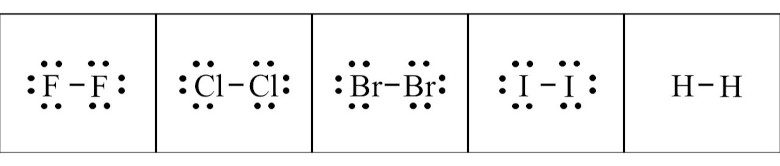
The diatomic elements oxygen and nitrogen have a bit more complex structure. There is a double bond between the two O atoms in the O2 dot structure. And there is a triple bond between the two N atoms in the N2 dot structure.
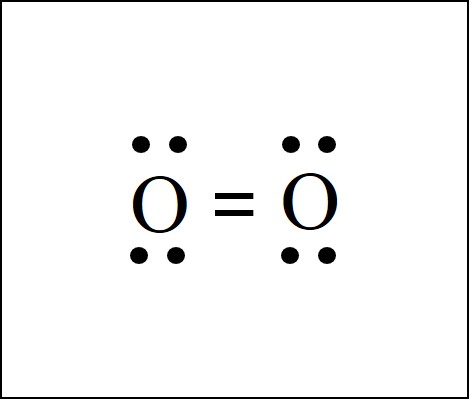
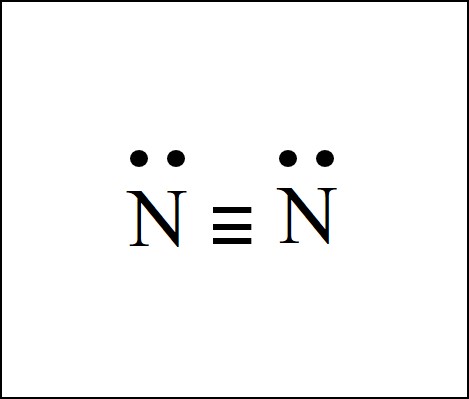
Diatomic Element Nomenclature
What Does "Oxygen" Mean?
The nomenclature, or naming system, for the diatomics is admittedly somewhat lacking.
Oxygen is the letter O on the periodic table. But, oxygen is also the stuff in the air that you breathe to stay alive. The air has O2, and there are not any lone O atoms floating around the atmosphere. Letter O is the element oxygen on the periodic table, but the oxygen here on planet Earth, the gas in the atmosphere, is O2. The logic here is that O represents an oxygen atom, but the atoms live in pairs to make O2 oxygen gas.
We can be specific by saying “O atoms” versus “oxygen gas.” O atoms are just O, whereas oxygen gas means O2. Still, most of the time people just say “oxygen” and you need to decide if they mean O or O2. Arg. If they are talking about atoms or the periodic table, they probably mean O. But if they are talking about an actual substance they probably mean O2. It could go either way, potentially, but it’s usually pretty obvious which is which based on the context.
The atmosphere also has lots of nitrogen. It says N on the periodic table. But the stuff in the atmosphere is N2. Likewise, a canister full of hydrogen gas would contain H2, even though the periodic table just says H. When they put chlorine in the pool, it’s Cl2 (not Cl). You need to know that this happens for all 7 diatomic elements.
No Lone O's
Diatomic means that an atom cannot exist by itself. There is no such thing as lone O atoms floating around the atmosphere. Oxygen exists as O2, because the O atoms must live in pairs. These atoms are too unstable to exist alone.
We can have O bonded with another atom to make a molecule, though. An O can bond with a C to make carbon monoxide gas CO. Oxygen atoms (O) always need to bond to something else. They can never be alone. The same goes for all 7 diatomic elements.
