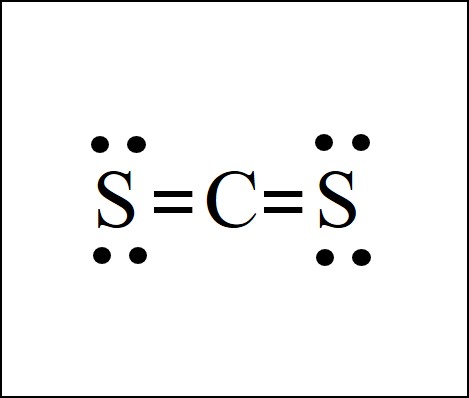A Lewis dot structure is a drawing of a molecule. The drawing only “works” for stable molecules that actually exist. So it’s a nice tool to explore how atoms bond into more complex substances.
A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram. When people mention Lewis or dots, they are talking about drawing a molecule to show how the atoms bond.
Download and print the black and white pdf. It’s 5 printer-friendly pages. There’s an answer key too in the other pdf file.
Follow the below examples to learn this important technique for drawing Lewis dot diagrams.
Drawing Lewis structures is based on the octet rule. The idea is that each atom (aside from H, hydrogen) is surrounded by 8 valence electrons. Each bond (stick) represents a pair of valence electrons, and the pairs of dots also represent pairs of valence electrons.
It’s all based on valence electrons, which are shown as dots. So Lewis structures are also called dot diagrams, electron dot structures, or Lewis dot diagrams.
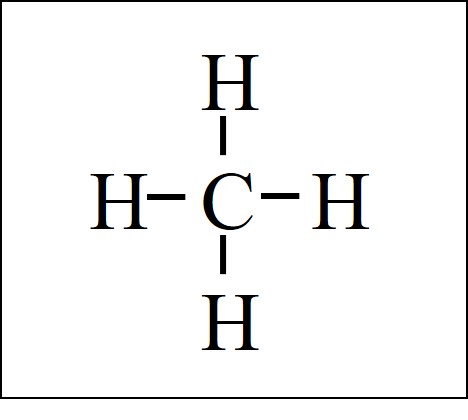
The CH4 Lewis structure has the typical case of carbon C in the center with 4 bonds to 4 other atoms. Note that hydrogen only forms 1 bond. So CH4 is the only combination that works…. there is no such thing as CH3 or CH5, for example, as it has to be CH4 when a carbon atom bonds with hydrogens. Methane is also called natural gas, and it’s the smallest of the alkane hydrocarbon family of molecules.
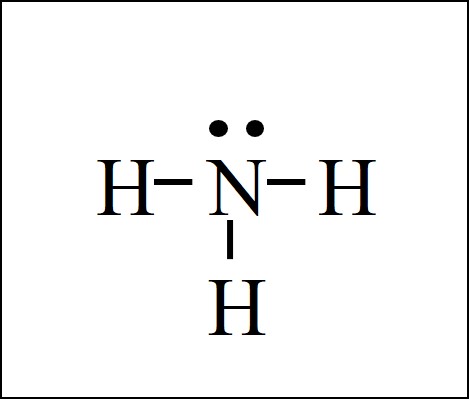
The NH3 Lewis structure has the typical case of nitrogen N in the center with 3 bonds to 3 other atoms. There is also a lone pair of electrons (unbonded electrons) on N. The lone pair (dots on N) could be drawn in any position… up, down, left, or right. Normally, however, we draw N with a pair of dots on top.
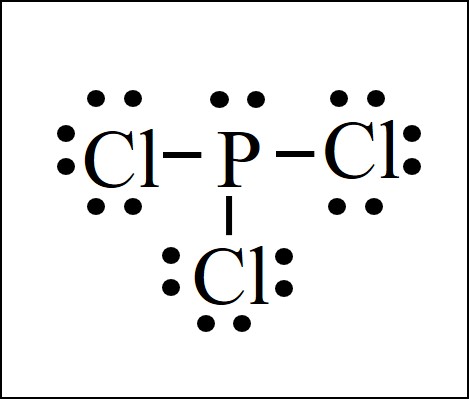
The PCl3 Lewis structure has the typical case of phosphorus P in the center with 3 bonds to 3 other atoms. Phosphorus is from the same column as nitrogen in the periodic table, meaning that P and N generally have the same bonding structure. Note the lone pair (dots without bonds) on top of P, just like for N in the previous example for NH3.
Chlorine Cl is a halogen that forms 1 bond. It’s much like hydrogen H in forming 1 bond, except halogens will be surrounded by 3 pairs of dots in addition to the bond.
The H2O Lewis structure has the typical case of oxygen O in the center with 2 bonds to 2 other atoms. Note the pair of lone lone pairs (two pairs of dots without bonds, or 4 dots total) on the top and bottom of O.
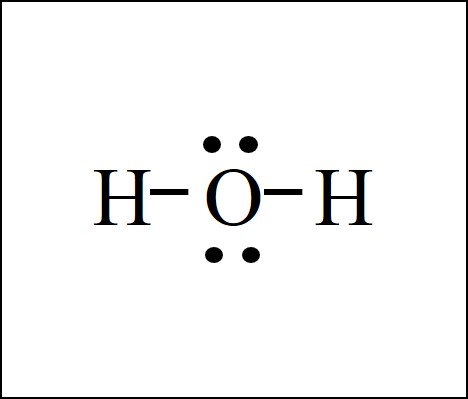
The H2S Lewis structure has the typical case of sulfur S in the center with 2 bonds to 2 other atoms. Sulfur is from the same column as oxygen in the periodic table, meaning that S and O generally have the same bonding structure. Note the pair of lone lone pairs (two pairs of dots without bonds, or 4 dots total) on the top and bottom of S.
One major difference between S and O is, that under certain circumstances not shown here, sulfur S may form 6 bonds.
The oxygen gas Lewis structure is a bit different than those above. First, there is no center atom to which the others are attached. It is just a pair of oxygen atoms, and oxygen gas O2 is known as a diatomic element. Oxygen O always needs 2 bonds, and in this case it bonds twice to another O atom. This is called a double bond, represented by two parallel lines or sticks. Note that there are still 2 pairs of dots (4 dots total) around each O atom, just like when oxygen forms single bonds as in the water example above. It is typical for oxygen to form double bonds.
The nitrogen gas Lewis structure is just a pair of nitrogen atoms, and nitrogen gas N2 is another diatomic element. Nitrogen N always needs 3 bonds, and in this case it bonds thrice to another N atom. This is called a triple bond, represented by three parallel lines or sticks. Note that there are still a pair of dots above each N atom, just like when nitrogen forms single bonds as in the NH3 example previously. It is typical for nitrogen to form triple bonds, and it may also have double bonds.
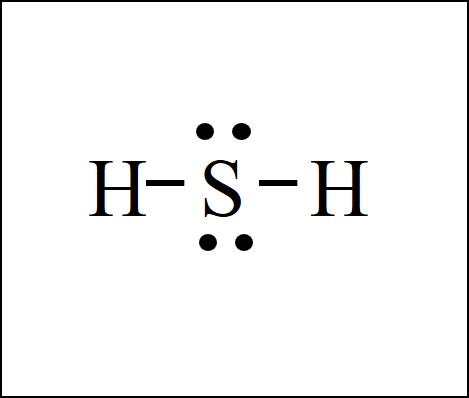
The CH2O Lewis structure has a central carbon atom C connected to 3 other atoms. Carbon always needs 4 bonds and oxygen always needs 2 bonds, so a double bond must form between C and O. The remaining two bonds for carbon C go to the 2 hydrogen H atoms. This is a typical case in which carbon is usually found in the center, with all other atoms connected to carbon.
This is the same as the H2CO Lewis structure or the HCHO Lewis structure, as there are a few ways to write out the formula for this chemical known as formaldehyde.
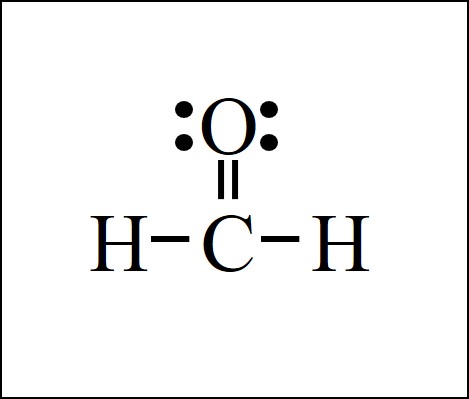
The carbon dioxide Lewis structure has carbon bonded to just 2 other atoms, both oxygen O atoms. There needs to be double bonds from carbon to each oxygen to ensure that carbon gets 4 bonds total and each oxygen gets 2 bonds total.
The CS2 Lewis structure is fundamentally no different than for CO2 in the previous example. Sulfur S and oxygen O are in the same column of the periodic table, so they both need 2 bonds and can double bond as needed.