
Use this naming polyatomic ions list and worksheet (answers provided) to quickly learn important chemical names and formulas. There are 4 exercises to practice, plus complete instructions, in the 5 page packet.
You can use the packet to quickly review names and formulas for polyatomic ionic compounds. You’ll learn the patterns easily, the names and formulas will become obvious, and you’ll save tons of time with chemistry class once you know this system. Before getting started, you should understand simple ionic compounds as shown in the Naming Ionic Compounds Worksheet.
Download and print the black and white pdf. It’s 5 printer-friendly pages. There’s an answer key too in the other pdf file.
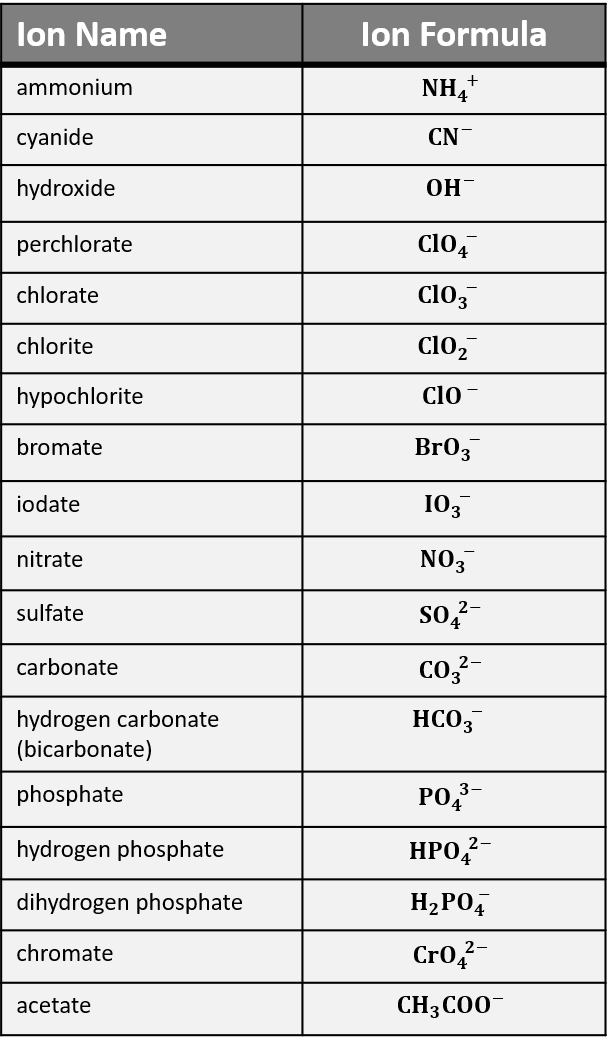
Polyatomic ions are charged groups of atoms. An example is ammonium ion, NH4+. It has five atoms (one nitrogen and four hydrogens) that share a charge of +1. The polyatomic ions remain intact, and parentheses may be required when using subscripts. For example, ammonium chloride is NH4Cl and ammonium sulfide is (NH4)2S. Ammonium is the only polyatomic cation. Common anions are shown in Table 1.
There are many polyatomic anions. Many occur in families of names. Start by learning the polyatomic ions ending with “-ate” such as chlorate (ClO3–), nitrate (NO3–), sulfate (SO42-), carbonate (CO32-), and phosphate (PO43-).
The corresponding “-ite” ion name has one less oxygen and the same charge. For example, chlorite ion is ClO2–. Less commonly used names are the “per__-ate” and “hypo__-ite” forms to indicate different numbers of oxygen.
Key in on the chlorate family in Table 1 to construct names for other ions. For example, sulfite (not in the table) would be SO32-, because it has the same charge and one less oxygen than sulfate (SO42- in the table).
Notes:
Polyatomic ions are NOT in the periodic table. Everything about ionic bonding still applies, except that you cannot figure out the charges by looking at the periodic table like for simple ionic compounds.
You need to either memorize, know, or have the list of polyatomic ions to figure out the names and formulas. There is simply no way to figure out the charges and numbers of oxygen atoms using the periodic table.
We suggest you become familiar with “the five -ates:” chlorate (ClO3–), nitrate (NO3–), sulfate (SO42-), carbonate (CO32-), and phosphate (PO43-). Many, many common chemicals in labs and chemistry classes are related to these five.
Complete the table of neutral ionic compounds with the formulas and names for each cation-anion pair.
For example, calcium ion Ca2+ and carbonate CO32- combine in a 1:1 ratio as the neutral compound calcium carbonate CaCO3, the major component of limestone formations.
Sometimes parentheses are used around polyatomics. For example Ca2+ and hydroxide OH– combine in a 1:2 ratio as the neutral compound calcium carbonate Ca(OH)2. The parentheses indicate that there are two hydroxides for each calcium.
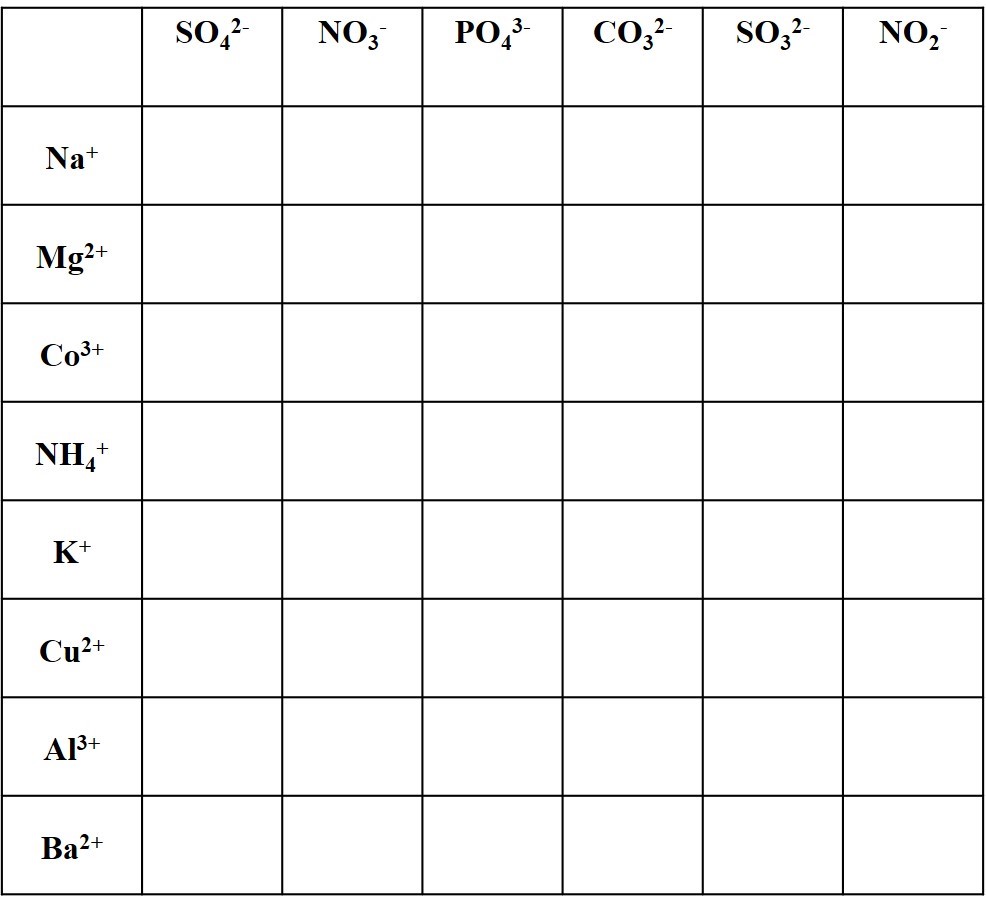
Provide the formula for each compound. If you don’t understand the Roman numerals, see the Naming Ionic Compounds Worksheet.

Provide the formula for each compound. If you don’t understand the Roman numerals, see the Naming Ionic Compounds Worksheet.

Provide the formula for each compound.
